Answer
1579.2 liters of H₂
Step-by-step explanation
Given that:
N₂ (g) + 3H₂(g) → 2NH₃ (g)
What to find:
The liters of H₂ required to produce 47 moles of NH₃.
Step-by-step solution:
Step 1: Determine the moles of H₂ required to produce 47 moles of NH₃.
Using the mole ratio of H₂ to NH₃ in the given reaction; 3:2
If 3 moles of H₂ = 2 moles of NH₃
So x moles of H₂ = 47 moles of NH₃
To get x, cross multiply and divide both sides by 2 moles of NH₃
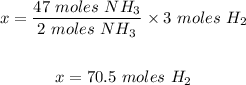
Step 2: Convert 70.5 moles of H₂ to liters.
Conversion factor: 1 mole of any gas at STP = 22.4 liters.
1 mole of H₂ = 22.4 liters
70.5 moles of H₂ = (70.5 mol/1 mol) x 22.4 liters = 1579.2 liters.
Therefore, 1579.2 liters of H₂ are required to produce 47 moles of NH₃.