Explanations:
Given the formula for the condition expressed as:
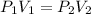
where:
• P1 and P2 are the ,initial and final pressure ,respectively
,
• V1 and V2 are the, initial and final volume, respectively
a) If the pressure is doubled, then the final pressure P2 will be 2P1
Substituting P2 = 2P1 into the formula, we will have:
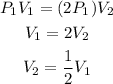
This shows that the volume will be halved if the pressure is doubled.
b) If the volume is tripled, this means that V2 = 3V1. Substituting the volume will give:
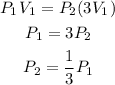
Hence if the final pressure will be one-third of the initial if the volume is tripled.
c) If the volume is reduced to one-fourth of its original value, then the new volume will be given as:
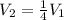
Substituting this into the formula:
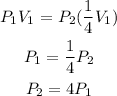
This shows that if the volume is reduced to one-fourth of its original value, the pressure will be quadrupled