Answer:
0.49moles
Explanations:
According to the ideal gas equation

where:
• P is the ,pressure ,of the gas
,
• V is the ,volume ,of the gas
,
• n is the ,number of moles
,
• R is the ,Gas constant
,
• T is the ,temperature, (in Kelvin)
Given the following parameters at STP
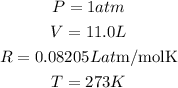
Determine the required number of moles
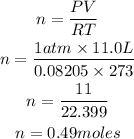
Hence the number of moles present in 11.0L of neon gas at STP at 0.49moles