Assuming the mass we have is only of HCl, not the whole solution, we can do the following steps:
- Use the molar mass of HCl to see how many moles of HCl correspond to 4.5g of HCl.
- Use the concentration of 1.5 M to see how much volume have the calculated number of moles of HCl.
To calculate the molar mass of HCl, we need the molar masses of H and Cl, which we can get from a periodic table:
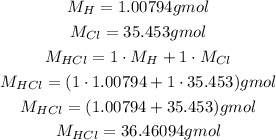
So, using it, we can calculate the number of moles of HCl in 4.5g of HCl:
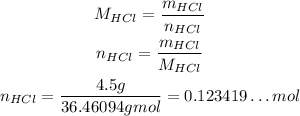
Since concentration is number of moles of solute divided by the volume of solution, we have:
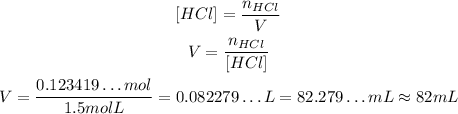
So, to get 4.5 g of HCl from a solution of 1.5 M of HCl, we need approximately 82 mL.