You can see that in the reaction 2 moles of HF react with 1 mole of Sn. We can do a rule of three, but first, we need to do the conversion from grams of Sn to their moles using the molar mass of Sn where you can see it in the periodic table which is 118.7 g/mol. The conversion would be:
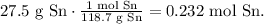
Now, use this result to state the rule of three:
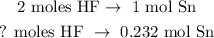
The calculation will be:
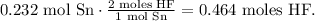
The answer is that 0.464 moles of HF are required to react with 27.5 grams of Sn (0.232 mol Sn).