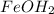The first step is to take the given percents as if they were masses.
It means, we are going to use 62.2g of Fe, 35.6g of O and 2.2g of H.
Convert the given mass to moles using the corresponding molecular mass:
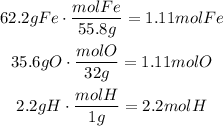
Now, divide every result by the smallest result of them, it means divide each result by 1.11:
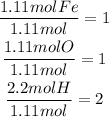
The obtained quotients will be the subscripts for each element in the empirical formula. It means that the empirical formula: