Answer
The mass of the metal = 28.8 g
Step-by-step explanation
Given:
Specific heat of the metal = 0.780 J/g⁰C
Quantity of heat required, Q = 45.0 J
Temperature raise, ΔT = 2.00 ⁰C
What to find:
The mass (m) of the metal.
Step-by-step solution;
Using Q = mcΔT, the mass (m) of the metal can be calculated.
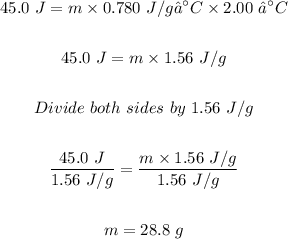
Therefore, the mass of the metal is 28.8 g.