Step 1
Gas (dioxide gas - NO2) is assumed to be ideal. Therefore, it is applied:
p x V = n x R x T
p = pressure = 650 mmHg (p will be converted because R will be 0.082 atm x L/mol K)
So, 1 atm = 760 mmHg => 650 mmHg x (1 atm/760 mmHg) = 0.86 atm
V = volume = 6.3 L
n = number of moles = mass NO2/molar mass NO2 = 10.0 g/46 g/mol = 0.22 moles
(Molar mass NO2 = 46 g/mol)
R = gas constant = 0.082 atm x L/mol K
T = temperature = unknown
-------------------
Step 2
T is cleared:
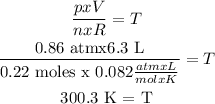
Answer: T = 300.3 K