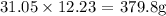Answer:
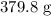
Step-by-step explanation:
Here, we want to get the mass of methylamine that must be combusted
From the question, we have the following:
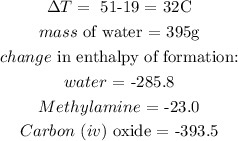
Now, we write the equation of reaction as follows:
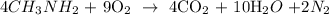
We have the overall change in enthalpy as follows:
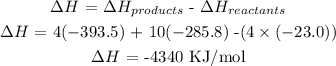
What this simply means is that 4340 KJ/mol of heat is released when 4 moles of methylamine is burned
Mathematically:
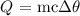
m is the mass of the methylamine we want to calculate
c is the specific heat capacity (For water, it is 4.2)
change in temperature is delta theta
What we want to calculate here is the amount of heat gained by the water
We have that as:
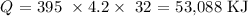
Now, we proceed to get the number of moles of methylamine burned actually
That would be:
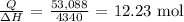
Finally, to get the mass, we multiply the number of moles by the molar mass
The molar mass of methylamine is 31.05 g/mol
Thus, we have the mass as: