Answer:
Step-by-step explanation:
Here, we want to select the option that represents the electronic configuration of an element in the excited state
When an element is in the excited state, electrons are promoted
In the excited state, the last electron would reside in a higher energy orbital
This is the case seen in the following configuration:
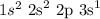
There is a promotion from the 2p to the 3s orbital