Answer
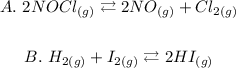
Leftward shift
System A; Decrease container size
No shift
System B; Increase conatiner size
System B; Decrease conatiner size
Rightward shift
System A; Increase container size.
Step-by-step explanation
Note: When there is an increase in volume, the equilibrium will shift to favor the direction that produces more moles of gas. However, when the area of the container has increased, there will be fewer collisions per unit area and the pressure will decrease. Volume is inversely proportional to pressure, if the number of particles and the temperature are constant.
Therefore, when there is decrease in container size, the pressure is increased, and the position of equilibrium moves in the direction of the fewest moles of gas and vice-versa.
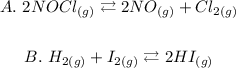
Leftward shift
System A; Decrease container size
No shift
System B; Increase conatiner size
System B; Decrease conatiner size
Rightward shift
System A; Increase container size.