1) Balance the chemical equation
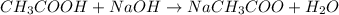
2) Moles of NaOH in the reaction
Convert mL into L
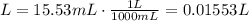
Find moles of NaOH
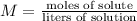
Plug in values and solve for moles
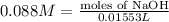
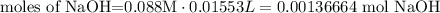
3) Moles of acetic acid that reacted with 0.00136664 mol NaOH
Molar ratio
1 mol NaOH: 1 mol CH3COOH
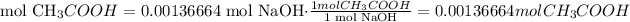
4) Convert moles of CH3COOH into grams of CH3COOH
The molar mass of CH3COOH is 60.05 g/mol
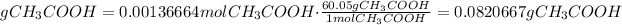
The mass of acetic acid is 0.0820667g
5) Percent of acetic acid
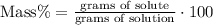
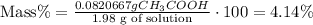
The percent of acetic acid by mass is 4.14%.
.