Answer:
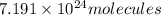
Explanations:
According to the Avogadros constant, 1 mole of an atom is equivalent to 6.02*10^23 molecules
Given the following parameter
Moles of CO2 = 11.945 moles
Convert the moles to molecules
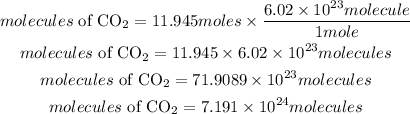
Hence the number of molecules present in 11.945 moles of CO2 is 7.191 * 10^24 molecules