Chemistry => Solutions => Preparing solutions
We are asked to find the final concentration of the solution that was diluted to 4.5L. For this we will apply the following equation that must have consistency in the units:
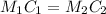
Where,
M1 is the initial molar concentration, it will have units of mol/L. M1=16.0mol/L
V1 is the initial volume it will have units of liters or milliliters. V1=205mL
We have to convert milliliters into liters, so we take into account that 1L=1000mL. So, we have:
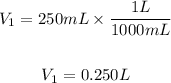
M2 is the final molar concentration, unknown
V2 is the final volume, 4.5L
So, the final molar concentration will be:
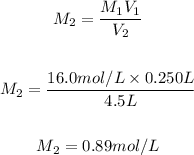
Answer: c. 0.89 mol/L