Answer:
There are 2.847094088x10^22 atoms.
- Prefix (green): 2.847094088
- Exponent (yellow): 22
Step-by-step explanation:
To calculate the number of atoms in 0.568g of diamond, it is necessary to convert the mass to atoms using the carbon molar mass (12.01g/mol) and the Avogadro's number (6.02x10^23 atoms/mole):
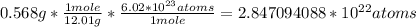
So, there are 2.847094088x10^22 atoms.