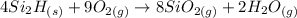Let's balance the equation:
To balance the equation, we first need to count the number of atoms of each element before and after the reaction occurs as you can see in the picture above.
As you can see, all elements are not balanced, so the first step that we could take is to balance "Si". To do this, remember that we can change the coefficients but we can't change the subscripts. In this case, we notice that there are 2 atoms of Si in the reactants side and there is 1 atom of Si in the products side, so we could multiply by 2 and count again:
Now, using this process three times more balancing H and O, we obtain the balanced reaction:
The answer is: