Answer:
198 g of water (H2O).
Step-by-step explanation:
What is given?
Mass of methane (CH4) = 88 g,
Molar mass of CH4 = 16 g/mol,
Molar mass of water (H2O) = 18 g/mol.
Step-by-step solution:
First, let's convert 88 g of CH4 to moles using its molar mass:
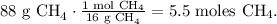
In the chemical equation you can see that 1 mol of CH4 reacted produces 2 moles of H2O, so let's see how many moles of H2O can be formed by 5.5 moles of CH4, as follows:
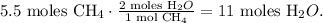
The final step is to convert 11 moles of H2O to grams using the molar mass of H2O, like this:
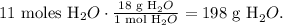
The answer would be that we can produce 198 g of water (H2O) by 88 g of methane (CH4).