Answer
4.76 mol Fe
Procedure
Consider the following balanced equation and determine the limiting reagent using the coefficients method
2Al(s) + Fe₂O3(aq)--> Al2O3(aq) + 2Fe(s)
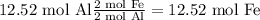
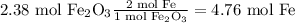
The lowest amount is the one produced by the iron (III) oxide therefore that is the limiting reagent and 4.76 mol of Fe is the max amount that can be produced of Iron.