The half life formula is :
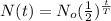
where N(t) = remaining quantity after t years
No = Original Quantity
t = time in years
T = half life in years
From the problem, we have :
N(t) = 30 grams
No = 100 grams
T = 674
Solve for t :
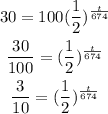
Take ln of both sides :
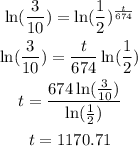
The answer is t = 1170.71 years