Answer
The mass ratio for H2O to O2 in the given reaction is 9 : 8
Step-by-step explanation
Given equation:
2H2 + O2 -----> 2H2O
What to find:
The mass ratio for H2O to O2 in the given reaction.
Solution:
The first step is to calculate the molar mass of H2O and O2.
From the periodic table, the atomic masses of (H = 1.0, O = 16.0)
So the mass of H2O in the reaction is 2(2 x 1 + 16) = 2(18) = 36.0 g
The mass of O2 in the reaction is (2 x 16) = 32.0 g
The final step is to determine the mass ratio for H2O to O2.
The mass ratio for H2O to water in the given reaction can be calculated as follows
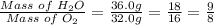
Therefore, the mass ratio for H2O to O2 in the given reaction is 9 : 8