1) List the known and unkown values
Initial conditions
Volume: 1.7 L
Pressure: 1.2 atm
Temperature: 20ºC
Final conditions
Volume: 1.1 L
Temperature: 90ºC
Pressure:
2) Set the equation
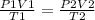
3) Convert Celsius to Kelvin
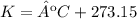
Initial temperature
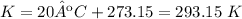
Final temperature
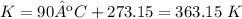
4) Plug in the known values
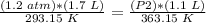
.
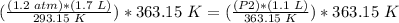
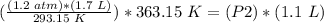
.
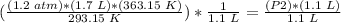
.
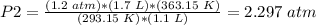
The new pressure in the coffee thermos is 2.3 atm.
.