ANSWER
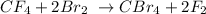
STEP-BY-BY STEP EXPLANATION:
Given equation
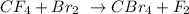
To balance the above equation, we will need to apply the law of conservation of mass
The next process is to assign variables to each of the reactants and products

For carbon
we have 1 carbon atom at the reactant side and 1 carbon atom on the product side
Mathematically
a = c
For Fluorine
We have 4 atoms of fluorine at the reactant side and 2 atoms of fluorine at the product side
4a = 2d
For Bromine
we have 2 atoms of bromine on the reactant side and 4 bromine atoms on the product side.
2b = 4c
Since a = c
Therefore, a = 1 and c = 1
2b = 4c
Recall that, c = 1
2b = 4
Divide both sides by 2
2b/2 = 4/2
b = 2
we also know that, 4a = 2d
a = 1
4(1) = 2d
4 = 2d
Divide both sides by 2
4/2 = 2d/2
d = 2
Therefore,
a = 1
b = 2
c = 1
d = 2