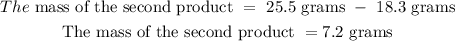Answer
7.2
Step-by-step explanation
The chemical reaction can be denoted as shown below
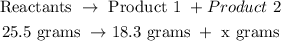
According to the law of conservation of mass which states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. This means the mass of the products in a chemical reaction must equal the mass of the reactants.
This implies that