Answer:
Step-by-step explanation:
Here, we want to get the product of the reaction
From what we have, the reaction between the two gives a precipitate, which is a solid product that is insoluble. This reaction is an example of a double displacement reaction
We have the balanced equation of reaction as follows:
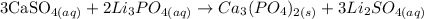
The precipitate is called Tri Calcium Phosphate