1 ) Balance the chemical equation
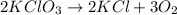
2) Convert grams of potassium chlorate into moles of potassium chlorate.
The molar mass of KClO3 is 122.55 g/mol.
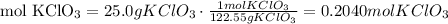
3) How many moles of KCl are produced from 0.2040 mol KClO3.
The molar ratio between KCl and KClO3
2 mol KCl:2 mol KClO3
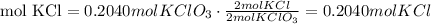
4) Convert moles of KCl into grams of KCl
The molar mass of KCl is 74.55 g/mol
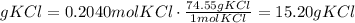
15.20 g KCl will be produced.
.