Answer
Mass of AlCl3 formed = 75.12 g
Step-by-step explanation
Given:
2Al + 3Cl2 --> 2 AlCl3
Mass of aluminum = 15.2 g
Mass of chlorine = 39.1 g
Required: How many grams of AlCl3 forms
Solution
Aluminum
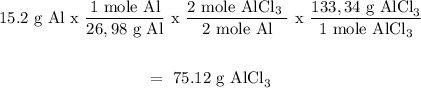
Chlorine
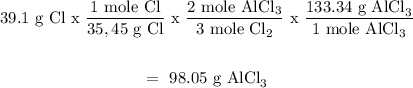
Aluminum is the limiting reagent because it produces less mass of AlCl3. Therefore the mass of AlCl3 that will be formed = 75.12 g