The reactants are Sr(OH)2 (aqueous) and Li3PO4 (aqueous).
The produces would be LiOH (aqueous) and Sr3(PO4)2. Remember that the product is obtained by exchanging cations and anions between the reactants.
So, the balanced chemical equation would be
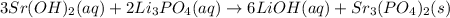
As you can observe, the product is aqueous and solid. The solid product is strontium phosphate. Additionally, remember that balancing a chemical equation refers to having the same number of atoms of each element on each side, that's why we add coefficients that multiply each element.