Answer:
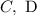
Step-by-step explanation:
Here, we want to select the correct answer choices
An exothermic reaction is one in which energy is given off or released to the surroundings. Now, let us evaluate each of the given options:
a) This is incorrect
It is incorrect because it is only during bond formation that energy is being released to the surroundings/environment
b) This is incorrect
Conversely, energy is released to the surroundings
c) This is correct
The enthalpy change for an exothermic reaction is negative
d) This is correct
When bonds are formed, energy is released to the surroundings
e) This is incorrect
The bond energy for the reactant is greater than that of the product in an exothermic reaction. This is why we have the enthalpy change as negative