Answer
0.46 moles
Step-by-step explanation
Given:
Mass of iron (III) oxide sample = 74 g
What to find:
The amount (mole) of iron (III) oxide in a 74 g sample.
Solution:
The amount (mole) of iron (III) oxide in a 74 g sample can be calculated using the mole formula.
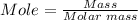
Using the molar masses of Fe = 55.845 g/mol, and O = 15.999 g/mol.
The molar mass of Fe2O3 = (2 x 55.845) + (3 x 15.999) = 159.687 g/mol
Putting mass = 74 g and molar mass = 159.687 g/mol, the amount will be
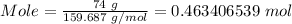
Therefore, the amount of iron (III) oxide in a 74 g sample is approximately 0.46 moles.