Answer:
The number of moles of argon = 7.835moles
Appropriate ratio = 1mole/22.4L
Explanations:
To get the number of moles of argon, we will use the conversion rate as shown:
1 mole = 22.4 L
Convert 175.5 liters of argon to moles
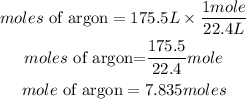
The equivalent number of moles of 175.5 liters of argon is 7.835 moles and the appropriate ratio is 1/22.4L