You have to graph the following function
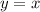
This is a linear function with slope m=1, this means that each time x increases one unit, y also increases one unit.
To sketch this function you have to choose at least two values of x and determine the corresponding value of y.
Then plot the points and draw the line.
I will make a table with 5 points of the line:
Now what's left is to plot all points and link them with a line: