Answer:
8
Explanations:
The empirical formula of a compound is defined as the simple whole ratio of atoms present in a compound.
Given the following parameters
• Moles of carbon = 1.3 moles
,
• Moles of hydrogen = 10.4 moles
,
• Moles of Oxygen = 10.4 moles
Calculate the simple whole ratio of each atom by dividing the mole number by the smallest mole value:
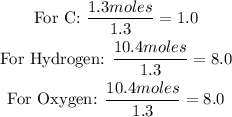
Determine the empirical formula:
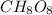
From the resulting empirical formula, the subscript for oxygen in the correct empirical formula is 8.