ANSWER
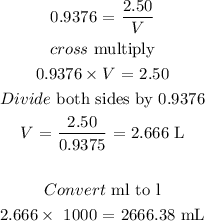
The volume is 2666.38L
Step-by-step explanation
Given information
The concentration (Molarity ) of H2SO4 is 2.50 M
The mass of NaOH is 75.0g
To find the milliliters of 2.50M of H2SO4, follow the steps below
Step 1: Write a balanced equation for the reaction
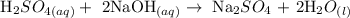
From the above-balanced reaction, you will see that 1 mole of the acid reacts with 2 moles of the base to give 1 mole of the salt and 1 mole of water
Step 2: Find the mole of sodium hydroxide using the below formula
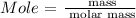
Recall that, the molar mass of NaOH is 39.997 g/mol
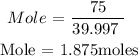
From the calculations, you will see that the mole of NaOH is 1.875 moles
Step 3: Calculate the mole of sulfuric acid using a stoichiometry ratiO
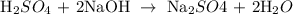
1 mole of H2SO4 is equivalent to 2 moles of NaOH
Let x represent the number of moles of H2SO4
Mathematically,
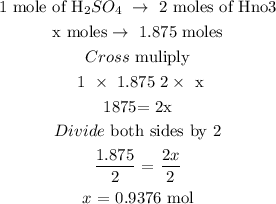
Hence, the mole of H2SO4 is 0.9376 moles
Step 4: Find the volume of the solution using the below formula
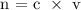
Where,
n = is the number of moles
C = concentration in molarity
V = Volume of the solution in Liters