Using the formula for change in energy,
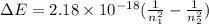
where n1 and n2 are 4 and 2 respectively.
Let's calculate the change in energy when this electron makes this transition.
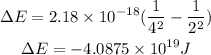
This negative value in the energy indicates that the electron jumps from a higher energy level to a lower energy level as a result of loss of energy.
From this, we can calculate the wavelength of the electron using the combination of Einstein's equation and Heisenberg's equation
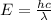
Where h = Plank's constant
c = speed of light
substitute the values into the equation and solve for the wavelength
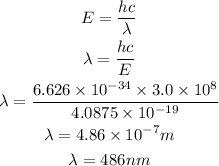
From the calculations above, the wavelength of the electron is 486nm