The balanced equation is:
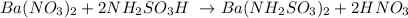
The chemical equation is balanced when the total amount of elements in the reactants side is the same as in the products side.
So, in this case, in the reactants side there are:
- 1 Ba
- 4 N
- 12 O
- 6 H
- 2 S
And in the products side there are also:
- 1 Ba
- 4 N
- 6 H
- 2 S
- 12 O