ANSWER
99.83 grams
Step-by-step explanation
The function that describes the amount of sample (in grams) after t years of decomposition is:
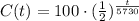
To get the amount of carbon 14 after 14 years, we calculate C(14) as following:
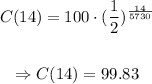
This way, we'll have 99.83 grams left.