Step 1 - Writing the chemical equation
The reaction that is taking place in this experiment is:
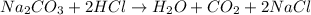
Note the proportion between the reactants is:
one mole of Na2CO3 reacts with 2 moles of HCl
Step 2 - Calculating the concentration of Na2CO3 in mol/L
The molar concentration of Na2CO3 can be calculated by the following formula:
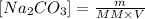
In this equation, m is the mass, MM the molar mass and V the total volume of solution.
We know that:
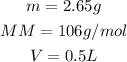
So, setting the values in the formula:
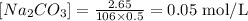
Step 3 - Finding how many moles of Na2CO3 reacted
Now let's find how many moles there were in 20ml of this solution:

Step 4 - Finding how many moles of HCl reacted
Let's remember that one mole of Na2CO3 reacts with 2 moles of HCl. Therefore, if 0.001 moles of Na2CO3 reacted, 0.002 moles of HCl also reacted.
Step 5 - Calculating the concentration of HCl in mol/L
The 0.002 moles that reacted were present in 18.5ml of HCl solution. Therefore:
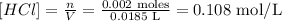
Answer: 0.108 mol/L