ANSWER
The total pressure of the gas inside the tank is 119 atm
Step-by-step explanation
Given that
The partial pressure of O2 is 45 atm
The partial pressure of N2 is 9 atm
The partial pressure of He is 65 atm
Follow the pressure below to find the total pressure of the mixture
Dalton's law of partial pressure states that the total pressure exerted by a mixture of gases is equal to the summation of the partial pressure of the individual gases.
Mathematically,
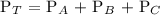
Substitute the given data into the formula above
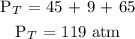
Therefore, the total pressure of the gas inside the tank is 119 atm