1) Write the chemical equation.
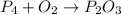
2) List the know and unknown quantities.
Reactants
P: 4
O: 2
Products
P: 2
O: 3
3) Balance P.
![P_4+O_2\operatorname{\rightarrow}2P_2O_3]()
Reactants
P: 4
O: 2
Products
P: 4
O: 6
4) Balance O.
![P_4+3O_2\operatorname{\rightarrow}2P_2O_3]()
5) Moles of P2O3.
The molar ratio between P4 and P2O3 is 1 mol P4: 2 mol P2O3.
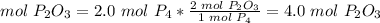
If 20 mol P4 are used, 4.0 mol P2O3 are formed.
.