Answer:
38.95 grams
Explanation
The formula for calculating the molarity of a substance is expressed as:
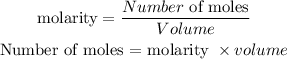
Given the following parameters:
Molarity = 0.3236 M
Volume = 352mL = 0.352dm³
Get the moles of the sugar using the formula
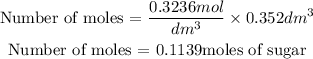
Next is to find the mass of the sugar present in the drink. This can be gotten using the formula below;
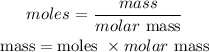
Get the molar mass of the sugar (C₁₂H₂₂O₁₁)
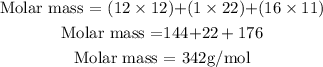
Get the required mass
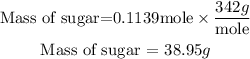
Hence the mass of sugar in the drink is 38.95 grams