Answer:
Actual yield = 6.78g
Theoretical yield = 13.85g
% yield = 48.95%
Explanations:
The balanced equation that results from the reaction of Almunimum and copper(II) sulfate is expressed as shown:
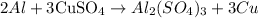
From the balanced equation, we can see that 2 moles of Aluminum produced 3 moles of copper.
Get the number of moles of aluminum;
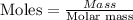
Mass of Al = 3.92g
Molar mass of Al = 26.98g/mol
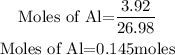
If 2 moles of Al produced 3 moles of Cu, then 0.145 moles of Al will produce;
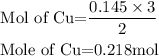
The calculated mass of copper will be the theoretical yield as shown:
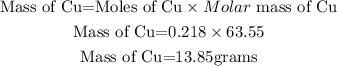
The theoretical yield is 13.85 grams
The actual yield is the given mass of copper which is 6.78 grams
Get the percentage yield

Hence the percent yield is 48.95%