ANSWER
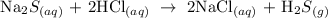
Step-by-step explanation
Given that
The two compounds reacting are sodium sulfide and hydrochloric acid
Molecular formula is defined as a chemical formula that gives the total number of atoms of each element in each molecule of a substance.
To write the molecular formula of the two compounds, apply the law of conservation of mass
Law of conservation of mass states that matter can neither be created nor destroyed but can be transformed from one form to another.
This implies that, the total number of atoms in the reactant sides is equal to the total number of atoms on the product side.
Hence, we have
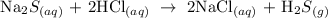
Therefore, option A is the correct answer