Answer:
The temperature is 336.9K.
Step-by-step explanation:
To calculate the temperature of CO2, it is necessary to replace the values of CO2 molar mass (0.044kg/mol), gas constant (R=8.314J/mol.K) and in this case the root mean square velocity (4.37x10^2m/s) in the root mean square velocity formula:
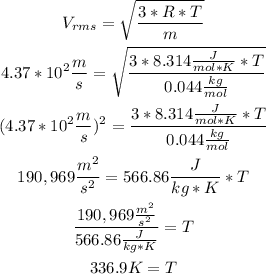
So, the temperature is 336.9K.