Answer:
2 moles of H2 gas will be produced.
Step-by-step explanation:
1st) It is necessary to write the balanced reaction:
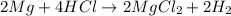
2nd) From the balanced equation we can find the moles of H2 gas that will be produced.
So, from the reaction, 2 moles of H2 gas will be produced.