The half life of carbon tells us how long it takes to get to half the original value. Thus, we can discover the decaying parameter of the exponential function, by doing the following:
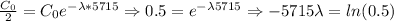
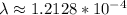
So, in order to find out how long it took to get to 42%, we can write is as the following:
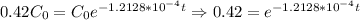
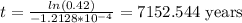
Thus, our answer is 7153 years