ANSWER
Step-by-step explanation
Given that:
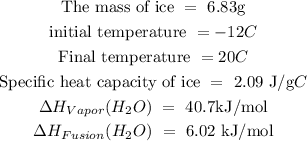
To find the heat required to convert ice to water, follow the steps below
Step 1: Write the heat formula
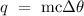
Since, the ice will be converted to solid first, the the final final temperature will be 0 degrees Celcius
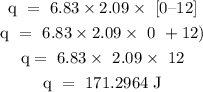
Step 2: find the heat required to convert the solid to liquid from 0 degrees Celcius to 0 degrees Celcius
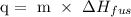
Where m is the mass of the sample
![undefined]()