The question asks us to identify the element formed when americium-241 (Am-241) emits an alpha particle.
Considering the alpha radiation, a particle containing 4 protons and 2 neutrons is emitted:
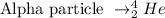
We can write the alpha decay of Am-241 as:
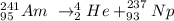
which means that americium-241 (Am-241) lost an alpha particle (He-4) and formed neptunium-237 (Np-237).
Therefore, the atomic symbol of the nuclide daughter formed when americium-241 undergoes an alpha decay is Np (option A).