Answer
¹⁷₉F → ¹⁷₈O + ₊₁⁰e
Step-by-step explanation
Note that a positron is the opposite of an electron.
Therefore, a nuclear chemical equation for the radioactive decay of fluorine-17 by positron emission is given below:
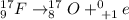
The atomic of fluorine is 9 and the mass number of the radioactive fluorine is 17, after the positron emission, it gives a different atom of oxygen.