To find the enthalpy of reaction of the given reaction, we have to use the following formula:
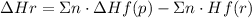
Where n is the coefficient of each substance, Hf(p) and Hf(r) are the enthalpies of formation of the products and the reactants respectively and Hr is the enthalpy of reaction.
The enthalpies of formation of each substance are:
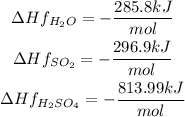
The enthalpy of O2 is 0 since it is a pure compound.
Replace for the given values and find the enthalpy of reaction:
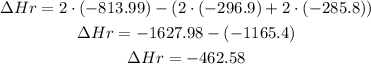
It means that the enthalpy of reaction is -462.6 kJ.