To answer this, we wil need to use the molar mass of Water, M.
Let m be the mass of water and n be the number of moles of water, then:
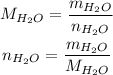
To calculate the molar mass of water, we need the molar mass of H and O, which we can get on a periodic table:
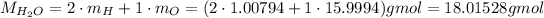
So:
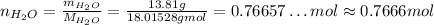
So, there are approximately 0.7666 mol of water.